Wastewater monitoring is conducted by the state for certain areas of Oregon. The data shows where the virus is detected in small- to medium-sized communities around the state.
Currently there are 29 communities participating on the project. A map shows if COVID-19 is “detected” or “not detected” in a community. It is important to note that if the virus was “not detected”, it does not mean that the community is free of COVID-19. Instead, it means that the virus may still be present in the area but below detection levels.
The monitoring serves as an “early warning” system to tell us if COVID-19 is spreading silently in communities. It is meant to help public officials try to prevent potential outbreaks or, if necessary, move resources to a community. OHA launched the project in the early fall with funding from the CDC.
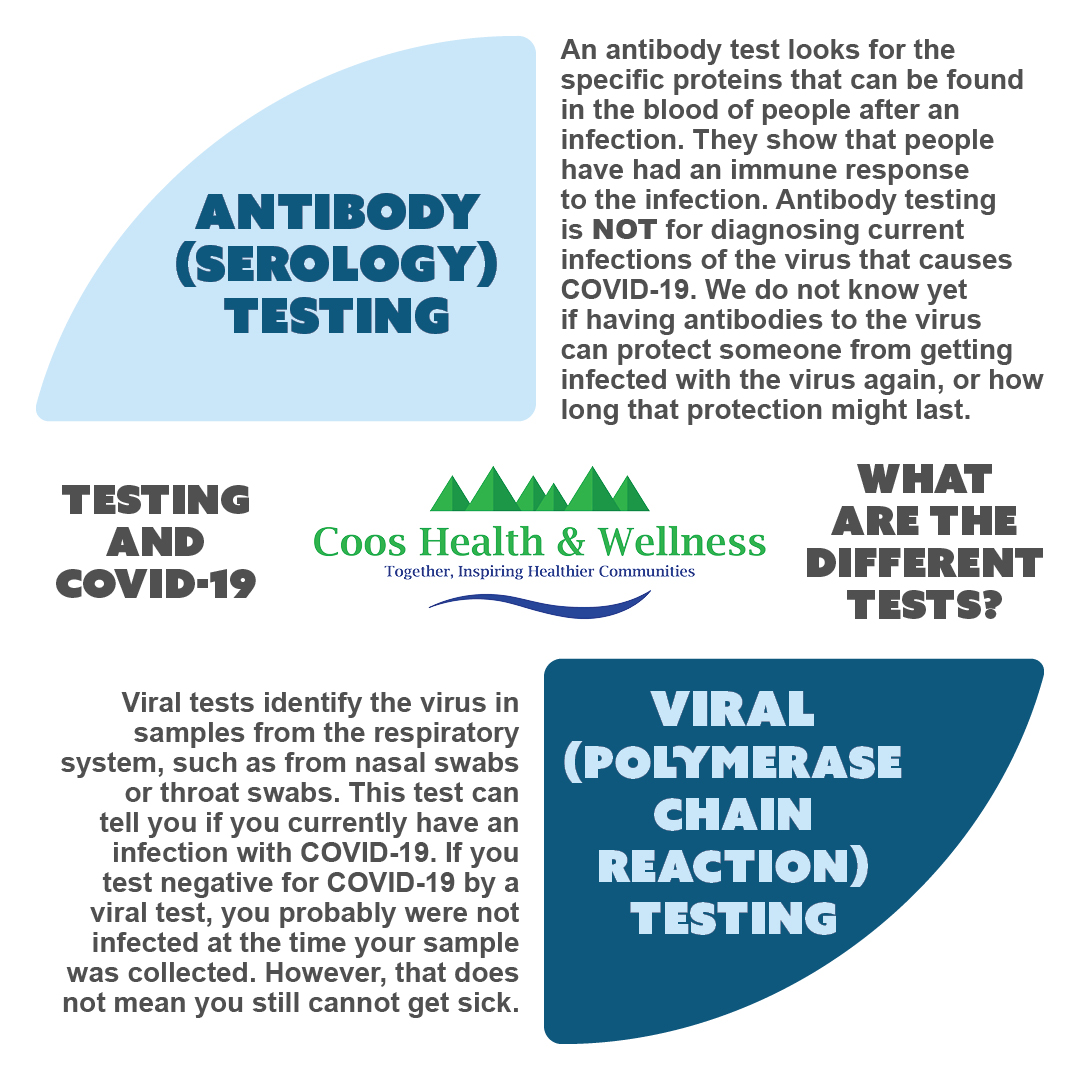
COVID-19 Testing
COVID-19 Vaccine Information
You can check with your medical provider as many area providers, including pediatricians, do have vaccines available. Vaccine appointments can be made at the following locations:
- Coquille Valley Hospital: 541-396-3101
- Southern Coos Hospital: 541-347-2426
- Bay Area Hospital: https://bayareahospital.org/covid-19/
- Coos Health & Wellness: 541-266-6700
- Broadway Pharmacy: 541-449-9190
- North Bend Medical Center: 541-266-1788
- Coast Community Health Center: 541-347-2529
- Nova Health North Bend Clinic: https://www.novahealth.com OR 541-305-4224 option #2
- Safeway
- Walmart
- Walgreens
- Rite Aid
- Fred Meyer
- Broadway Pharmacy: please call 541-449-9190 to schedule
You may be wondering what the difference is between an Emergency Use Authorization (EUA) and approval from the U.S. Food and Drug Administration (FDA). We’ll use this article to explore the topic in general, but we’ve posted a longer version on our blog with more details.
FDA Approval
In the case of an FDA approval, the FDA analyzes data on “the safety, efficacy, purity and potency” of every vaccine through a rigorous testing process, which usually begins with testing in animals.
If animals experience the intended effect of vaccination without concerning side effects, then the testing will continue in human trials, which are carried out in three phases.
If the clinical trials are considered a success, the FDA will also evaluate the vaccine manufacturing processes in place to ensure the vaccine can be made safely and consistently.
Following testing, FDA scientists and medical professionals carefully review all collected data and decide whether the vaccine is sufficiently safe and beneficial for use. If the vaccine meets these criteria, it is distributed for use in the United States and monitored closely thereafter.
When a vaccine receives FDA approval, health care providers with prescribing rights may prescribe it to their patients as they see fit.
Emergency Use Authorization
The COVID-19 vaccines from Pfizer, Moderna and Johnson & Johnson have all received an Emergency Use Authorization (EUA) from the FDA. EUAs are granted only during a declared emergency like the COVID-19 pandemic.
Similar to the FDA approval process, vaccine studies for EUA are conducted across three phases. All three COVID-19 vaccines currently authorized for emergency use were studied in tens of thousands of volunteers from diverse backgrounds and regions, with data monitored by independent data safety monitoring boards.
If the trials indicate that the vaccine is safe and effective for use, the manufacturer may apply to FDA for EUA. Then, FDA scientists and physicians with expertise in vaccine research evaluate the data, alongside a group of public health experts who make up the Vaccines and Related Biological Products Advisory Committee (VRBPAC). That committee makes a recommendation to FDA.
Weighing all the evidence, along with the VRBPAC, the FDA decides whether the benefits of a vaccine are likely to outweigh its risks. If the vaccine meets these criteria, it is granted an EUA and distributed for use in the United States and monitored closely thereafter. The EUA specifies how the vaccine must be used and it is valid only for the duration of the declared emergency.
FDA approval and EUA are two different processes that the FDA can use to make a vaccine available. Both of them follow the rigorous safety standards set forth by the FDA.
Frequently Asked Questions
Flu vs. COVID-19
Symptoms:
Flu viruses can cause mild to severe illness, including common signs and COVID-19 symptoms.
COVID-19 seems to cause more serious illnesses, in some people. Other signs and symptoms of COVID-19, different from flu, may include change in or loss of taste or smell.
Spread:
Most people with the flu are contagious for about 1 day before they show symptoms. For those with COVID-19, it’s possible for people to spread the virus for about 2 days before experiencing signs or symptoms and remain contagious for at least 10 days after signs and symptoms first appeared.
Complications:
Both COVID-19 and flu can result in complications, including:
- Pneumonia
- Respiratory failure
- Acute respiratory distress syndrome (i.e. fluid in lungs)
- Sepsis
- Cardiac injury (e.g. heart attacks and stroke)
- Multiple-organ failure (respiratory failure, kidney failure, shock)
- Worsening of chronic medical conditions (involving the lungs, heart, nervous system or diabetes)
- Inflammation of the heart, brain or muscle tissues
- Secondary bacterial infections (i.e. infections that occur in people who have already been infected with flu or COVID-19)
COVID-19 Additional Complications:
- Blood clots in the veins and arteries of the lungs, heart, legs or brain
- Multisystem Inflammatory Syndrome in Children (MIS-C)
Contact Us
Please feel free to reach out to us should you have any questions:
Por favor, no dude en commincarse con nosotros si tiene alguna pregunta:
Mitigation Measures
ENFORCEMENT & COMPLIANCE RESOURCES
Provisional Guidance and Information for Healthcare Providers
Information and Resources for the Public
- Tenant Protections in Oregon: English Spanish
- DHS Community Resource list
- Oregon Health Authority
- Building a Safe and Strong Oregon- Office of the Governor
- Oregon Health Authority Facebook Page
- Oregon Health Authority Facebook page en Español
- Centers for Disease Control and Prevention (CDC)
- Discouraging Discrimination Due to Actual or Perceived Exposure to COVID-19
- Grocery Delivery and Pickup Services in Coos County
COVID-19 Facts, & Information
COVID-19 Funeral Assistance | FEMA.gov
Here are a few high level bullet points from the website:
- In April, FEMA will begin accepting applications.
- FEMA is working to set up a dedicated phone number that can be used to apply for funeral assistance.
- The death must have occurred in the United States, including the U.S. territories, and the District of Columbia.
- The death certificate must indicate the death was attributed directly or indirectly to COVID-19.
- Additional guidance is being finalized and will be released to potential applicants and community partners as soon as possible. In the meantime, people who have COVID-19 funeral expenses are encouraged to keep and gather documentation.
Cleaning & Hygiene Information
- When and How to Wash Your Hands | CDC
- Coughing & Sneezing
- COVID-19 Disinfection Guidelines
- Cleaning and Disinfection for Households
- Disinfectants for Use Against SARS-CoV-2 (COVID-19)
Preventative Measures
- What Does it Mean to Self Isolate?
- ¿Qué Significa Autoaislarse?
- CDC Guidance: Use of Cloth Face Coverings to Help Slow the Spread of COVID-19
Food & Utilities Resources
- WIC Services during COVID-19
- Oregon Coast Community Action (ORCCA) COVID-19 Resources
- Meeting Basic Needs during COVID-19 – Coos Family Resources
- Food Hero – healthy recipes and meal ideas
- Coos County School Nutrition Program – COVID-19 Response
- Coos/Curry Emergency Food Resource Guide
Household Care & Resources
- Household Checklist to Prepare for COVID-19
- Child and Family Resources
- Intimate Partner Violence and Child Abuse Considerations During COVID-19
Accessibility Information & Resources
- Wheelchair and Assistive Technology Users – Precautions for COVID-19
- Deaf or Hard of Hearing Communication Card for COVID-19
- Unable to Speak Communication Card for COVID-19
- COVID-19 Resources for Families of Children and Youth with Special Health Care Needs
Health & Wellness
Apply for food, cash and other assistance from home. All Oregonians can apply for food, cash and child care assistance provided through the Oregon Department of Human Services (ODHS) from home without having to visit an office in person. To apply from home, visit govstatus.egov.com/or-dhs-benefits for information on how to apply for assistance using an online application, email, mail, telephone or application drop off. Oregonians who need urgent and ongoing food assistance can visit needfood.oregon.gov. For more ways to connect with ODHS or to find other types of assistance, contact 211info:
- By dialing 2-1-1 from any phone
- Text your zip code to 898211
- By email at help@211info.org
- 211info.org
- covid19.211info.org
- Mental Health and Coping During COVID-19
- Caring For Yourself Through a Pandemic
- 1-Minute Mindfulness Exercises
- Tips For Social Distancing, Quarantine, And Isolation During An Infectious Disease Outbreak
- Coping with Stress during the COVID-19 Outbreak
- Helping Children Cope with Stress during the COVID-19 Outbreak
Volunteer Opportunities
In coordination with the Emergency Operations Crew for Coos County, it is important to ensure that the most vulnerable people receive help and that all volunteers and recipients of support are safe. If you’re healthy and low risk and would like to help and volunteer, please email Angela Mayfield, Volunteer Coordinator, at: Angela.Mayfield@chw.coos.or.us or call 541-266-6703.
Emergency Fund For The Homeless
If you want to contribute to the work for the homeless task force for COVID-19 and ensure that people could self-isolate if they were positive with the virus, please go to: emergencyfundforthehomeless.com
Hand-sewn Face Masks
CDC Guidance: Use of Cloth Face Coverings to Help Slow the Spread of COVID-19
Bay Area Hospital is accepting donations of sewn face masks (hospital-approved patterns can be found on their Facebook page) from the general community.
Donations can be delivered to the screening stations at either the main hospital entrance or at the Emergency Room entrance between the hours of 7:00 am and 5:00 pm.
Donations are also being collected at Coos Head Co-Op located at 353 S 2nd Street in Coos Bay. They are open Monday-Friday from 9:00 am to 7:00 pm, Saturdays from 9:00 am to 6:00 pm, and Sundays from 10:00 am to 6:00 pm.
Click HERE for hospital-approved face mask patterns from Bay Area Hospital Facebook page.
Informational Videos
Novel Coronavirus 2019 Public Service Announcements
Influenza A and RSV are on the rise while Covid19 remains a factor in the current illness wave
With the holiday season upon us and many we know falling ill, it is important to remember the preventative measures to keep yourself and the ones you love healthy for the holiday season. Wash your…
PSA: September is Suicide Prevention Month
National Suicide Prevention Week is September 4 to 10, 2022 Suicide is among the leading causes of death In Oregon and is a major public health concern. PLEASE SEE THE ATTACHED PSA FOR MORE INFO:…